Medical Device
Medical |
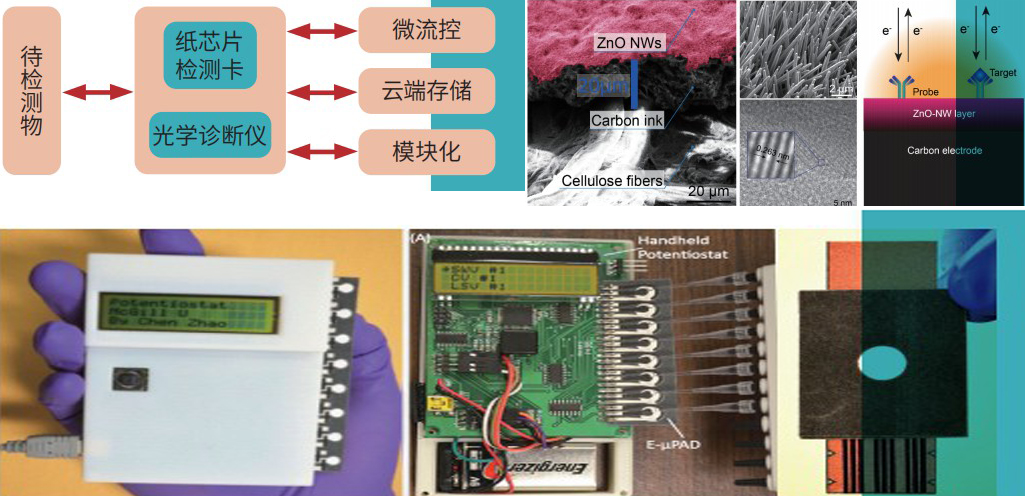
A Portable Paper-Based Microfluidic Device for In vitro Diagnostic
The products of this project are mainly used In the field of In Vitro Diagnostics (IVD). The portable, low-cost, fully automatic, modular paper-based diagnostic devices developed through the project adopts the international advanced microfluidic paper-based analytical technology to effectively implement automatic liquid control and disease detection on the micron-scale paper- based channel. The device adopts modular design, which can be widely used in various detection scenarios by installing different modules, such as rapid and accurate detection of influenza virus, heart markers, tumor markers, etc.
|
||
 |
 |
 |
|

|
Technical Parameter
|
APPLICATION PROSPECT | |
| Detection steps | Manual sampling and automatic detection |
·Screening of upper respiratory infectious diseases (detection of influenza A and B viruses)
·HIV, hepatitis B virus testing
·Cardiac marker testing
·Detection of tumor markers (lung cancer, liver cancer, etc.)
·Rapid detection of food ingredients, pesticide residues, pathogenic bacteria, heavy metals and other food safety
|
| Detection time | <20min | |
| sensitivity | >95% | |
| Specificity | >97% | |
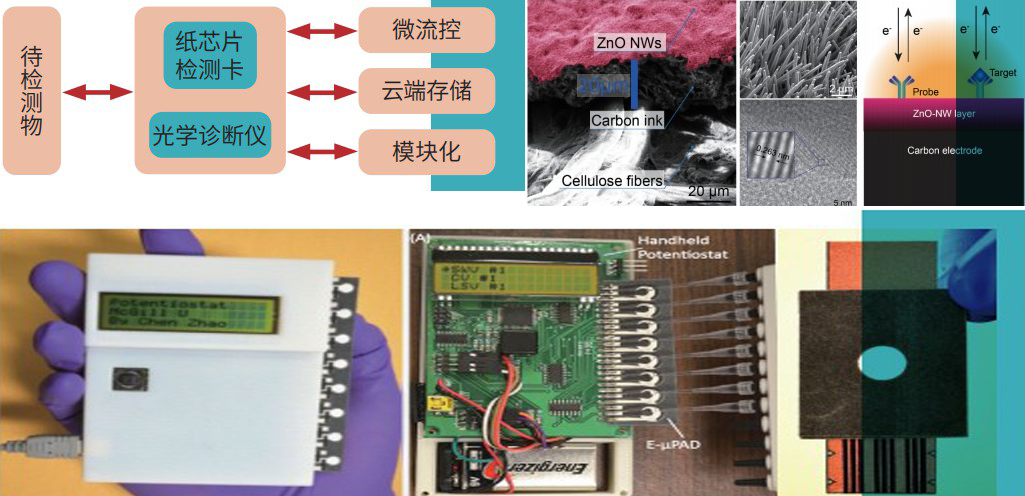

A Portable Paper-Based Microfluidic Device for In vitro Diagnostic
|
Technical Parameter
|
APPLICATION PROSPECT | |
| Detection steps | Manual sampling and automatic detection |
·Screening of upper respiratory infectious diseases (detection of influenza A and B viruses)
·HIV, hepatitis B virus testing
·Cardiac marker testing
·Detection of tumor markers (lung cancer, liver cancer, etc.)
·Rapid detection of food ingredients, pesticide residues, pathogenic bacteria, heavy metals and other food safety
|
| Detection time | <20min | |
| sensitivity | >95% | |
| Specificity | >97% |
|